By Adam West, Course and Curriculum Manager at CriticalPoint
Trending, misunderstanding, or both?
In conversations with pharmacy folks and certifiers, I’ve stumbled upon a potentially concerning trend. Is anyone doing smoke studies anymore? Many industry friends and acquaintances have admitted that the incidence of smoke studies in the pharmacy has decreased significantly, and certification companies aren’t providing this specific test as a service.
Certification companies I’ve spoken with reveal their rationale to be that smoke study testing is not part of the standard service in routine certification. To that point, (in some ways) they’re not wrong. Included in their rationale are liability fears, cost factors, time constraints, and limited participation of pharmacy staff.
Now, let’s clarify a few things… legitimate and valuable airflow smoke pattern testing sessions are a moderate-size production. This test shouldn’t be a quick and dirty thing. To properly perform this test it takes formal processes, visual and written documentation, allocated time, and most important, participation from the compounding staff.
CETA’s CAG-014 Airflow Visualization Study
The Controlled Environment Testing Association (CETA) recognized that smoke studies should have guidance that is more comprehensive and decided that this test should be separate from CAG-003 Certification of Sterile Compounding Facilities for USP Compliance.
It’s important to highlight that CETA’s guide on smoke studies does not specify a frequency in relation to certification. CAG-014 Air Visualization Study states that ISO Class 5 devices are tested “at least once, and repeated any time changes are made to the compounding process, as well as at any frequency required by regulatory bodies.” It does not specify a frequency congruent with a certification session.
Elements of performing proper “Airflow Visualization Studies (AVS)” per CAG-014 are as follows:
- Reporting and documentation — detailing specific camera types and angles, video editing, and capturing specific primary engineering control (PEC) data
- Tools, materials, and supplies — describing techniques and best practice aids for performing testing
- Focal points and areas of particular interest during simulated compounding – detailing critical sites, placement, and operator techniques to capture during test and video documentation.
- List of areas of concern within the PEC relevant to poor airflow characteristics
CETA CAG-014 is a performance guideline and supplements common regulatory requirements such as USP and local board of pharmacy inspectors. This is a valuable resource for certifiers and pharmacies performing airflow smoke pattern testing in-house. CriticalPoint recommends that pharmacies obtain the entire CAG library on their own that includes the CAG-014.
USP <797> Dynamic Airflow Smoke Pattern Testing
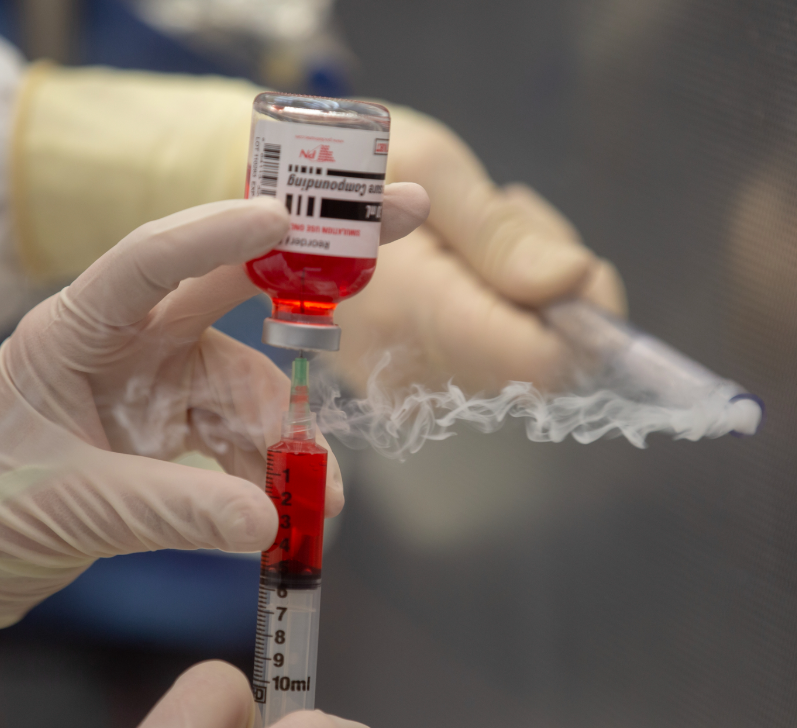
A dynamic airflow smoke pattern test must be performed in the PEC initially and at least every 6 months to ensure that 1) the laminar airflow system (LAFS) is properly placed into the facility, and 2) compounders understand how to utilize the unidirectional airflow to maintain first air in the direct compounding area (DCA).
- PEC must be initially verified by a dynamic airflow smoke pattern test to demonstrate minimal disruption in airflow.
- Smoke pattern tests must be performed for each PEC during dynamic operating conditions to demonstrate unidirectional airflow and sweeping action over and away from the preparation(s).
- Dynamic airflow smoke-pattern tests must be documented.
(Note: not a comprehensive testing list)
Under Section 5. Certification and Recertification, dynamic airflow smoke pattern testing is considered part of certification. Unfortunately, there is a potential to misunderstand what smoke study testing requires from a logistical standpoint. Pharmacies must plan accordingly for the certification process while maintaining operations and allowing certification services to occur.
The certification process takes a significant amount of time to perform properly. Value and quality may be reduced when combining smoke studies with the routine certification process because there’s an inherent rush to complete the testing in a timely manner.
Although considered part of certification, it may be more practical to plan airflow smoke testing during a separate session while still maintaining the six-month frequency as part of certification compliance.
What does this mean to the pharmacy?
Whether or not a particular certification company provides smoke studies, the pharmacy is responsible for ensuring this testing is performed at their facility during certification intervals . CriticalPoint strongly encourages that the pharmacy’s designated person work with reputable certification companies that include this USP-required test as part of routine certification.
CriticalPoint recommends that smoke studies be performed during operational process verification when performing staff training on media-fill, on compounding technique, and on use of automated compounding devices. Pharmacies and certification companies must work together to facilitate the testing process properly and compliantly.
Want to view all of our blogs all in one spot? Access our entire Chronicle library here!
Click here to learn about CriticalPoint’s Designated Person Administrative Best Practices – Virtual Training!
